KSEEB SSLC Science Solutions
Chapter 1 Carbon and its Compounds
LearnFatafat offers free Solutions for SSLC Science Chapter 1 Carbon and its Compounds. Chapter covers the topics like bonding in carbon,versatile nature of carbon, chemical properties of carbon compounds, important carbon compounds and more. Check video lessons, notes and MCQ quizzes for SSLC Science Chapter 1 Carbon and its Compounds click here to buy.
Solutions for SSLC Science Chapter 1 Carbon and its Compounds
1. Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds. (b) 7 covalent bonds. (c) 8 covalent bonds. (d) 9 covalent bonds.
Answer: (b) 7 covalent bonds
2. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid. (b) aldehyde. (c) ketone. (d) alcohol.
Answer: (c) ketone.
3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer: (b) the fuel is not burning completely.
4. Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer: Carbon neither loses nor accept 4 electron in outer shell which makes it unstable. However, carbon shares its four electron with other carbon atom, or atoms of other element. Thus, it forms covalent bonding. Therefore, bonding existing in CH3Cl. In CH3Cl carbon requires 4 electrons to complete its octet, hydrogen requires one electron to complete its duplet and chlorine requires one electron to complete its octet. Therefore, carbon shares its 3 electrons, one each with three hydrogens and one electron with chlorine. I.e. three bonds of carbon are formed with hydrogen atoms and one bond is formed with chlorine.
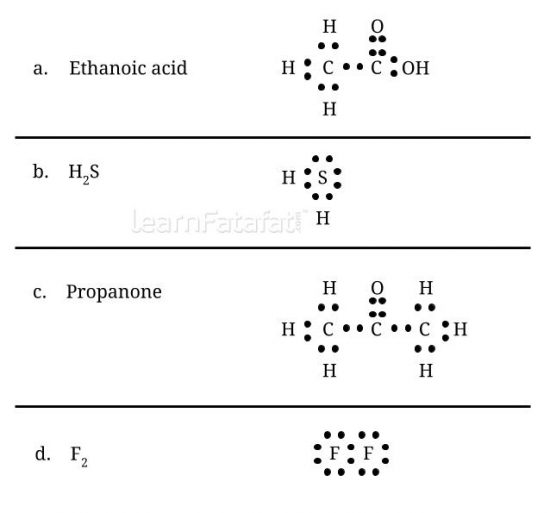
5. Draw the electron dot structures for
(a) ethanoic acid. (b) H2S. (c) propanone. (d) F2.
Answer:
6. What is a homologous series? Explain with an example.
Answer: Series of carbon compounds having same functional group but different number of carbon atoms is called as homologous series. Methane, ethane, propane,butane, pentane etc are examples of homologous series with general formula CnH2n+2. If observed carefully, there is difference of -CH2 in successive compound.
Methane – CH4
Ethane – C2H6
Propane – C3H8
Butane – C4H10
Pentane – C5H12
7. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Answer:
| Ethanol | Ethanoic Acid |
|---|---|
| Ethanol has pleasant odour | Ethanoic acid has smell like vinegar |
| Do not reacts with metal carbonates and metal hydrogen carbonates | Reacts with metal carbonates and metal hydrogen carbonates |
| Do not change the colour of litmus paper | Changes the color of litmus paper from blue to red |
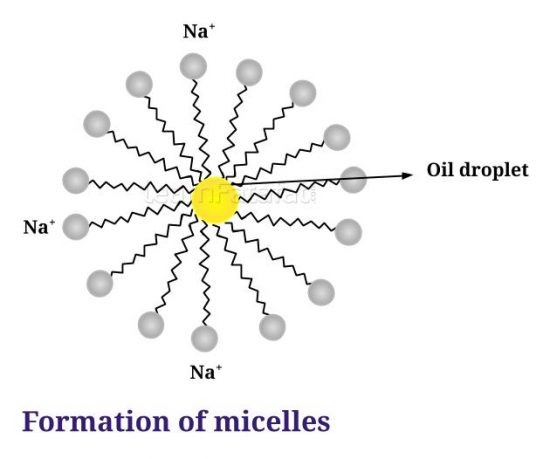
8. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Answer: The molecules of soap are sodium or potassium salt of long chain carboxylic acids. Soap molecules consist of two ends ionic-end also called hydrophilic end (Na⁺ or K⁺) and long chain of carboxylic acids called hydrophobic end. Most dirt is oily in nature hence when it comes in contact with soap molecules the carboxylic acid ends dissolves in dirt and get oriented inwards. while the hydrophilic ends get oriented away from the dirt forming a structure called micelle. Micelles did not come together because of ion-ion repulsion hence they are separated and can be easily rinsed with water. The soap micelles are large enough to scatter light hence soap solution appears cloudy.
9. Why are carbon and its compounds used as fuels for most applications?
Answer: Carbon and its compounds have high calorific value, and releases lots of light and heat energy when burnt. Therefore, these are used as fuels for most applications.
10. Explain the formation of scum when hard water is treated with soap.
Answer: Soaps are sodium or potassium salts of fatty acids. In hard water, soaps do not work properly. Hard water contains calcium and magnesium salts. When soap is added in hard water calcium and magnesium replaces sodium and potassium, thereby forming an insoluble substance called as scum.
11. What change will you observe if you test soap with litmus paper (red and blue)?
Answer: Soap turns red litmus paper blue whereas blue litmus paper remains blue. This suggest that soap is basic in nature.
12. What is hydrogenation? What is its industrial application?
Answer: Process of addition of hydrogen is called hydrogenation. Process of hydrogenation is used to add hydrogen in unsaturated hydrocarbon in presence of Ni catalyst to convert into saturated hydrocarbon. Hydrogenation is mostly used in vegetable oils, that contains long chains of unsaturated carbons.
13. Which of the following hydrocarbons undergo addition reactions: C2H6, C3H8, C3H6, C2H2 and CH4.
Answer: Unsaturated hydrocarbon undergo addition reactions. Therefore among the given examples unsaturated hydrocarbon C2H2 and C3H6 will undergo addition reactions.
14. Give a test that can be used to differentiate chemically between butter and cooking oil.
Answer: Butter is saturated fat, thus, it cannot be hydrogenated. Oil is unsaturated fat, therefore, it can be hydrogenated to saturated fats. On this basis, butter and cooking oil can be differentiated chemically.
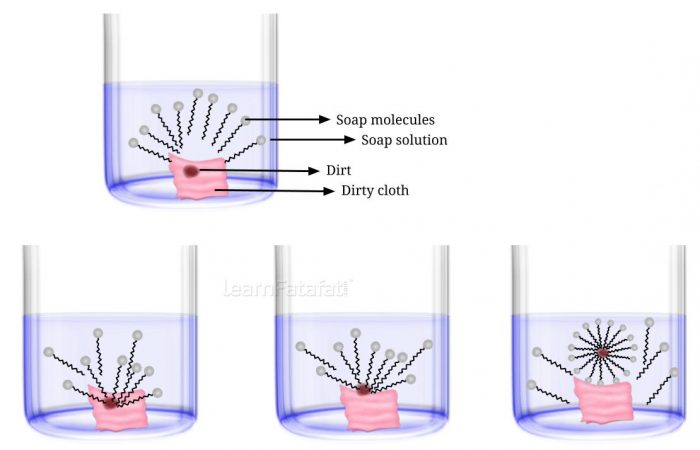
15. Explain the mechanism of the cleaning action of soaps.
Answer: The molecules of soap are sodium or potassium salt of long chain carboxylic acids. Soap molecules consist of two ends ionic-end also called hydrophilic end (Na⁺ or K⁺) and long chain of carboxylic acids called hydrophobic end. Most dirt is oily in nature hence when it comes in contact with soap molecules the carboxylic acid ends dissolves in dirt and get oriented inwards. while the hydrophilic ends get oriented away from the dirt forming a structure called micelle. Micelles do not come together because of ion-ion repulsion hence they are separated and can be easily rinsed with water. The soap micelles are large enough to scatter light hence soap solution appears cloudy.
KSEEB SSLC
Access SSLC video lessons online (internet required) or offline(internet not required) in SD Card, Pendrive, DVD, Tablet.
Chapter 1 – Carbon and its Compounds





